A Blinded, Randomized, and Placebo-Controlled Clinical Trial of a Novel Veterinary Product for the Management of Chronic Kidney Disease in Cats
Principle Investigator
Description
Evidence exists to suggest that aging of the kidney may contribute to the onset and progression of chronic kidney disease (CKD). Rapamycin is a drug that is a known modulator of the aging process and additionally may decrease the formation of fibrosis (scarring) in the kidney. A feline formulation of the drug is available, and this study aims to assess the potential benefit of the drug in cats with CKD.
Participation
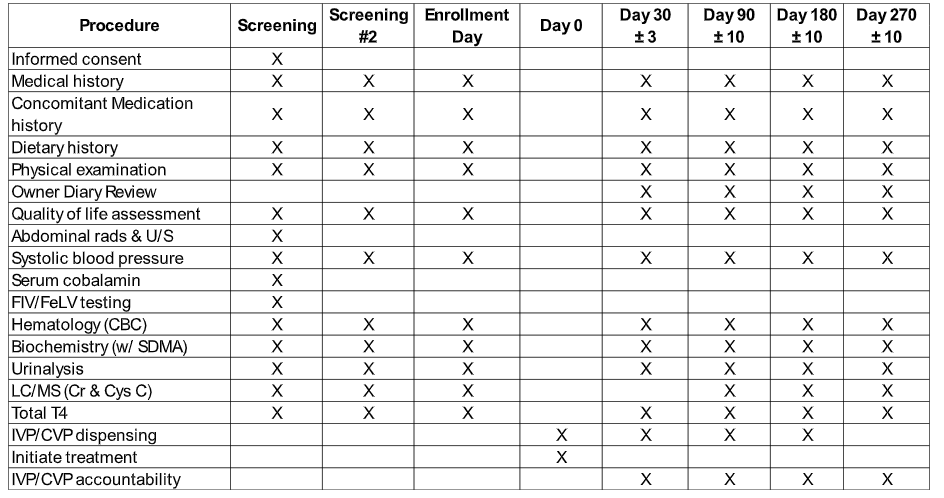
1. At the time of screening, your cat will receive a physical examination and comprehensive laboratory screening to confirm stage of CKD and exclude other newly diagnosed conditions (eg. hyperthyroidism, hypertension).
2. At one of the screening visits an abdominal ultrasound and radiographs will also be performed (these procedures typically require light sedation).
3. After confirmation of enrollment, your cat will be randomized to receive Rapamycin or placebo which will be administered orally as a whole tablet (followed with water or food to ensure swallowing) once weekly for ~40 weeks (270 days). Some cats may require two tablets per dose.
4. You will be asked to bring your cat back for four recheck visits (day 30, 90, 180 and 270) for a physical exam, blood and urine collection and blood pressure measurement.
A summary of events is presented in the table below:
Enrollment Start
12/15/2024
Enrollment Finish
02/01/2026
Eligibility
Inclusion Criteria:
- Cats diagnosed with IRIS Stage II or III chronic kidney disease
– Based on elevated serum creatinine (1.6 – 4.9 mg/dL) with USG < 1.035 - Cats must be amenable to pill administration
- Cats must be fed a stable diet for at least 28 days with a commitment to not substantially alter the diet during the study period.
- Cats must have a body weight ≥ 2.2 kg (~5lbs)
- Cats must ≥ 5 years and < 15 years of age
Exclusion Criteria:
- Congenital kidney disease (example: dysplasia or polycystic kidney disease), diabetes mellitus, uncontrolled systemic illnesses
- CKD complications include kidney infection or ureteral obstruction, moderate to severe anemia (PCV < 25%), decompensated CKD requiring hospitalization, and intravenous fluid therapy.
- Prohibited concurrent therapies include cyclosporine, cisapride, beta-blocking agents, antifungal agents, diltiazem, and other medications that could affect rapamycin concentrations.
- Other concurrent therapies such as dietary management, potassium supplementation, anti-hypertensive medications, and subcutaneous fluids are acceptable if they were initiated at least 28 days prior to enrollment and are given consistently throughout the study period.
Incentives:
There is no cost to you for enrolling your cat in this study. You will receive complimentary physical examinations for your cat and diagnostic testing (blood work, urinalysis, abdominal ultrasound and radiographs).
Flyer
Contact
For more information, please contact the Harris Lab at harrislab-clinicaltrials@ncsu.edu
- Categories:


