Efficacy of Potassium Citrate as a Treatment for Metabolic Acidosis in Cats with Chronic Kidney Disease
Principle Investigators
Autumn N. Harris and Shelly Vaden
Description
Chronic Kidney Disease (CKD) is defined as the presence of functional abnormalities in one or both kidneys over a prolonged period of time. CKD is the most common typeof kidney disease and management has largely been focused on supportive and symptomatic therapy, with very few new therapies available to treat or manage CKD that have been shown to improve outcomes. There is a critical need for research to identify new therapeutic targets for managing cats with CKD. Metabolic acidosis is a commonly observed complication in patients with kidney disease, occurring across all stages of the disease. Metabolic acidosis is known to have numerous deleterious consequences and, most importantly, has been linked to major adverse clinical outcomes and has been identified as a significant risk factor for CKD progression and mortality in patients with kidney disease. This study aims to evaluate potassium citrate in the management of metabolic acidosis in cats with CKD.
Testing Requirements
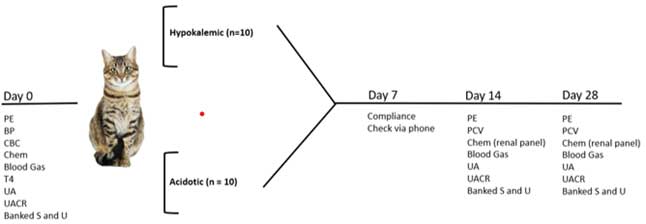
At the time of screening, your cat will receive a physical examination, comprehensive laboratory screening to confirm stage of CKD (complete blood count, chemistry panel, Total T4 urinalysis, UPC, blood gas, blood pressure measurement). After confirmation of enrollment, your cat will be randomized to either the hypokalemia or metabolic acidosis group and be prescribed potassium citrate which will be administered orally as a tablet twice daily for approximately 28 days. You will be asked to bring your cat back for two recheck visits (day 14 and 28) for a physical examination, blood and urine collection and blood pressure measurement.
A summary of anticipated events is presented in the diagram below. No changes in medication or diet should be made during the study without discussing first with the study team.
Enrollment Start
07/01/2025
Enrollment Finish
07/01/2026
Eligibility
Inclusion criteria:
- Cats already diagnosed with CKD – considered IRIS Stage 2-4
- No change in diet in 2 weeks (ideally one month)
- Other medication changes at the time of enrollment are acceptable as this may
significantly affect enrollment (e.g. if Mirataz were also prescribed), but these
cats may be excluded from final analysis. Amlodipine is an exception – it must
have been started at least 2 weeks prior due to its effect on potassium. - Cats must have hypokalemia or metabolic acidosis
o Hypokalemia is defined as serum potassium < 4.0 mEq/L
o Metabolic acidosis is defined as a serum bicarbonate <18 mEq/L
Exclusion criteria:
- The presence or strong suspicion for hyperaldosteronism; presence of severe diarhhea or *vomitting (* particularly if caused by concurrent uncontrolled disease other than CKD such as chronic enteropathy, pancreatitis, hepatobiliary disease, hyperthyroidism, etc.), proximal renal tubular acidosis, or other underlying medical conditions which could result in hypokalemia.
- Evidence of metabolic acidosis secondary to causes other than CKD (e.g. ketoacidosis, lactic acidosis, diarrhea, renal tubular acidosis, and ethlyene glycol toxicity).
- Concurrent use of ACEi, ARB, spironolactone, or other drugs which are known to have interactions with either potassium citrate and/or potassium gluconate.
Incentives
There is no cost to you for enrolling your cat in this study. You will receive complimentary physical examination for your cat and diagnostic testing (blood work and urinalysis).
Flyer
Contact
For more information, please contact the Harris Lab at harrislab-clinicaltrials@ncsu.edu
- Categories: